Example Problems
Small-Angle Scattering |
|||||||||||||||||||||||||||||||||||||||||
| 1 |
Two perfectly spherical latex particles of diameter d=10 nm form a rigid dimer. The two spheres touch, i.e., their centers are separated by a = 10 nm. The dimers are investigated by solution scattering.
How would the pattern change if the distance between the centers of the spheres were increased? How would the pattern change if the diameter d for each latex sphere were decreased for constant a? |
||||||||||||||||||||||||||||||||||||||||
| 2. |
Small Angle Scattering from rodlike molecules gives the following intensity distribution
Determine the radius of gyration for the scatterer’s cross section. Qualitatively, sketch the shape of the Guinier plot one would obtain if the particle were spherical. Comment on the contribution from the rods length and their cross-section to this graph. |
||||||||||||||||||||||||||||||||||||||||
| 3 |
Citrus Tristeza virus, a rod-like RNA-plant virus, is investigated by neutron solution scattering. In 50% D2O, the coherent contribution to the scattered intensity, I(q) with q=2 pi s, is measured as in Table 1. The corresponding values for the experiment performed in 37% D2O are listed in Table 2. Table 1.
Table 2
|
||||||||||||||||||||||||||||||||||||||||
| 4 |
Three different types of Guinier plots are used to determine some important dimensions of differently shaped molecules from small-angle scattering.
To determine the maximum dimension, assume that the particle is homogeneous and it has a simple geometric shape.
|
||||||||||||||||||||||||||||||||||||||||
Cryo-electron microscopy |
|||||||||||||||||||||||||||||||||||||||||
| 1. |
|
||||||||||||||||||||||||||||||||||||||||
| 2. |
|
||||||||||||||||||||||||||||||||||||||||
| 3. | Develop a simple expression for the magnification of the objective lens of an electron microscope, making use of the general solution to the relativistic trajectory equations for electrons moving close to the axis of a cylindrical magnetic lens. | ||||||||||||||||||||||||||||||||||||||||
| 4. |
Give an account of the way in which spherical aberration can affect the resolving power of the objective lens of an electron microscope. Suggest how theseeffects can be minimized and what criteria might finally limit such partial remedies. A specimen containing a point-to-point spacing of 0.27 nm is introduced into an electron microscopeobjective of numerical aperture 0.01. When operating with an accelerating voltage of 100 kV show that theoretically it should be possible to resolve a spacing of the given magnitude. If the objectivelens has a coefficient of spherical aberration of 2 nm, how will your argument and conclusion have to be modified? |
||||||||||||||||||||||||||||||||||||||||
| 5. |
Compare and contrast the physical principles that determine the resolution of the scanning electron microscope with those of the transmission instrument. Indicate how the resolution of the transmission instrument might be further affected by lens aberrations. |
||||||||||||||||||||||||||||||||||||||||
Crystallography |
|||||||||||||||||||||||||||||||||||||||||
| 1. |
(a) Explain the meaning of the following crystallographic terms:
(b) Draw a diagram for space group P21/c with the origin at the intersection of the 21 screw axis, and with the c-glide plane. Clearly make all the symmetry elements on the diagram. Redraw the space group diagram, placing the origin on the center of symmetry and label the symmetry elements. Write down the coordinates of the general equivalent positions. What special positions occur in this space group? What are the independent conditions limiting X-ray reflection for P21/c? |
||||||||||||||||||||||||||||||||||||||||
| 2. |
A crystal of the oxygen-binding protein haemoglobin was mounted on a precession camera with a crystal to film distance of 60 mm and a CuKɑ X-ray source with a wavelength of 1.5418 Å. Precession photographs of the a*b* and a*c* nets were recorded. On the photographs containing the hk0 reflections, the distances between the pairs of diffraction spots were: -18,0,0 and 18,0,0 = 34.76 mm; 0,-20,0 and 0,20,0 = 37.84 mm. The distance between the 0,0,-12 and 0,0,12 was 33.90 mm on the h0l photographs. Assuming the crystal was orthorhombic, determine the unit cell parameters, a,b, and c, and the unit cell volume in A3. The haemoglobin crystals contain four asymmetric units per unit cell, with one haemoglobin molecule per asymmetric unit, and have a density of 1.2 gcm-3 |
||||||||||||||||||||||||||||||||||||||||
| 3. |
Write a detailed account of one of the following topics:
|
||||||||||||||||||||||||||||||||||||||||
| 4. |
Explain the meaning of the following crystallographic terms
For each of the space groups: P21/C, Cm, Ibca:
Draw diagrams to show a set of general positions and the corresponding set of symmetry elements for space group Cm, and give the coordinates of the general equivalent positions. |
||||||||||||||||||||||||||||||||||||||||
| 5. |
Give a brief account of the oscillation, and historical Weissenberg and precession methods of X-ray data collection. Comment on the advantages and disadvantages of each method. A substance crystallizes in space group Pnma with four molecules per unit cell. Precession photographs of a crystal mounted about its c axis were taken on a camera with a crystal-to-film distance of 60.0 mm, using CuKɑ radiation of wavelength 1.5418Å. On the h0l photograph, the distances between the pairs of diffraction spots 200, -200 and 004, 00-4 were 68.27 mm and 99.47 mm, respectively. If the distance between the axial reflections 020 and 0-20 on the okl photograph was 92.97 mm, calculate the values of the unit cell parameters a, b, and c, and the volume of the unit cell, V, in Å3. Determine the molecular weight, Mr, of the substance is the crystal density is 8.965 g cm-3 and NAvog is 0.60221. |
||||||||||||||||||||||||||||||||||||||||
| 6. |
Write short notes on the following topics:
A compound containing platinum (Pt) and some lighter atoms, crystallizes with four Pt atoms in the unit cell with the following atomic positions
If the experimentally observed structure factors Fo(111) and Fo(333) are 191 and 281, respectively, calculate the percentage contribution to the structure factors due to the Pt atoms only, relative to the values observed for all atoms in the unit cell. The values of the atomic scattering factors fPt(111) and fPt(333) are 73.5 and 59.5, respectively. |
||||||||||||||||||||||||||||||||||||||||
| 7. |
Explain the meaning of the terms:
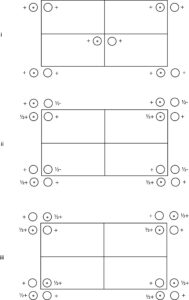
The space group diagrams illustrated below show a set of general equivalent positions in a unit cell. For each case:
|
||||||||||||||||||||||||||||||||||||||||
| 8. |
Outline with the aid of a suitable sketch, the concept of the reciprocal lattice in X-ray crytallography. Derive the Laue equations which define the direction of scattering of X-rays by crystalline solids. USi crystallizes in the orthorhombic system with a=5.65, b=7.65, and c=3.90 Å and z=4. The U atoms lie at positions
Calculate the structure factors (a) F(200) and (b) F(111). The contributions to the structure factors from Si may be neglected. The atomic scattering factors for U are tabulated below as a function of (sin θ)/ƛ.
|
||||||||||||||||||||||||||||||||||||||||
Multiple techniques
|
|||||||||||||||||||||||||||||||||||||||||